Publications
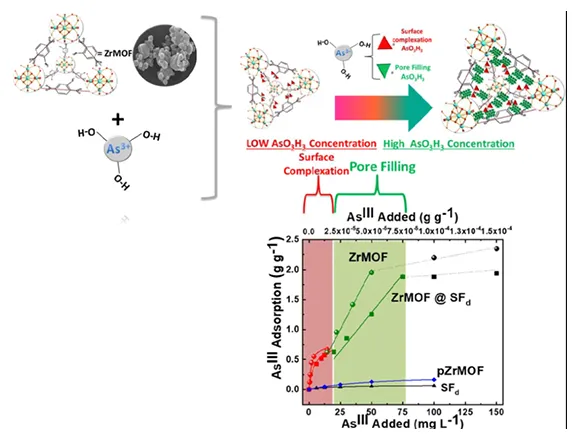
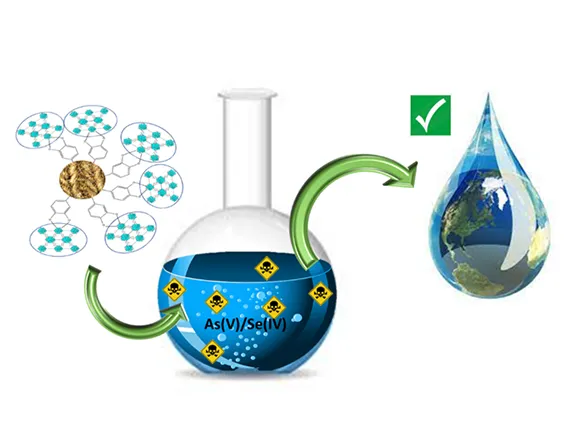
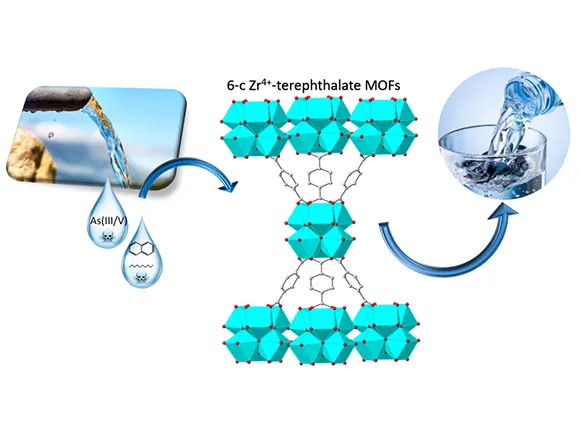
Exposure of humans to Arsenic from groundwater drinking sources is an acute global public health problem, entailing the urgent need for highly efficient/low-cost Arsenite (AsIII) up-taking materials. Herein we present an innovative hybrid-material, ZrMOF@SFd operating like an “AsIII-sponge” with unprecedented efficiency of 1800 mg AsIII gr−1. ZrMOF@SFd consists of a neutral Zirconium Metal-Organic Framework [ZrMOF] covalently grafted on a natural silk-fiber (SFd). ZrMOF itself exhibits AsIII adsorption of 2200 mg gr−1, which supersedes any -so far- known AsIII-sorbent. Using XPS, FTIR, BET-porosimetry data, together with theoretical Surface-Complexation-Modeling (SCM), we show that the high-AsΙΙΙ-uptake is due to a sequence of two phenomena:[i] at low AsIII-concentrations, surface-complexation of H3AsO3 results in AsIII-coated voids of ZrMOF, [ii] at increased AsIII-concentrations, the AsIII-coated voids of ZrMOF are filled-up by H3AsO3 via a partitioning-like mechanism. In a more general context, the present research exemplifies a mind-changing concept, i.e. that a “partitioning-like” mechanism can be operating for adsorption of metalloids, such as H3AsO3, by metal oxide materials. So far, such a mechanism has been conceptualized only for the uptake of non-polar organics by natural organic matter or synthetic polymers.